
Your Tactical Guide to Achieving PIC/S GMP Compliance for Global Market Access
In the global pharmaceutical market, compliance transcends mere regulatory adherence; it plays a pivotal role in facilitating international trade. A
We Embody a Legacy of Incredible Discoveries, Exploring the Boundaries of Innovation and Science

At Zenovel, we integrate our extensive expertise across a wide range of domains, from Good Clinical Practice to Regulatory Affairs, to present valuable insights and thought leadership. Each blog reflects our intense dedication to innovation, offering a forum to share our perspectives, groundbreaking ideas, and industry expertise. Through our blogs, we hope to uplift, inform, and support scientific, medical, and pharmaceutical innovation.



In the global pharmaceutical market, compliance transcends mere regulatory adherence; it plays a pivotal role in facilitating international trade. A

The pharmaceutical industry is facing a convergence of innovation and regulation as it approaches 2026, with GxP services playing a

The recent update of ICH E6(R3) marks a significant evolution in clinical trial execution, transitioning from a compliance-centric approach to
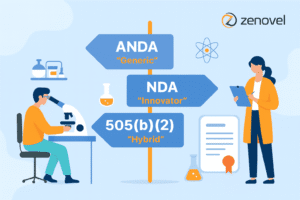
In the pharmaceutical industry, particularly in India’s expanding market, the regulatory pathway is a crucial determinant that influences the transition

In the current pharmaceutical environment, the integrity of a company’s supply chain relies heavily on its weakest link, particularly concerning
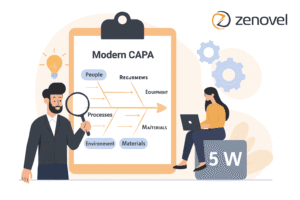
In clinical research, Good Clinical Practice (GCP) sets ethical and quality standards. Non-compliance during audits poses systemic risks to patient
We provide affordable, innovative, and high-quality solutions to pharma industry with ethics driven research and increasing accessibility to high quality medicine worldwide
Copyright © 2024 Zenovel. All rights reserved.