In the dynamic, rapidly changing environment of the pharmaceutical industry, product quality and regulatory compliance is mandated. Computer System Validation (CSV) is essential to that goal, since it demonstrates that computerized systems used in manufacturing, testing or managing data are reliable and compliant. This Zenovel guide gives an overview of the basic principles of Computer System Validation, the regulatory context, the essential process steps and best practices for ensuring pharmaceutical compliance in 2025.
Why CSV Matters in Pharmaceuticals
Pharmaceutical manufacturing heavily relies on computerized systems for control, data collection, and product consistency. These systems must function flawlessly to maintain quality and comply with global regulations. Computerized System Validation (CSV) is a structured approach to verify system performance, ensuring data integrity, product safety, and regulatory adherence. Implementing robust CSV practices can prevent costly errors, reduce compliance risks, and protect patient safety by ensuring consistent hardware, software, and processes.
Regulatory Framework for Computer System Validation
Compliance with regulatory standards is a cornerstone of Computer System Validation. Key regulations and guidelines include:
Figure 1: Regulatory Framework

The regulations emphasize the importance of documented evidence of validated, reliable, and GMP-compliant systems, as non-compliance can result in regulatory penalties, product recalls, or compromised patient safety.
Key Components of CSV
Computer System Validation comprises various components like hardware, software, networks, cloud services, and documentation, all of which undergo validation to ensure proper functionality and support the overall quality management system.
GAMP 5 Categories
The Good Automated Manufacturing Practice (GAMP) 5 framework categorizes systems into software and hardware types, each with specific validation methods:
Software Categories

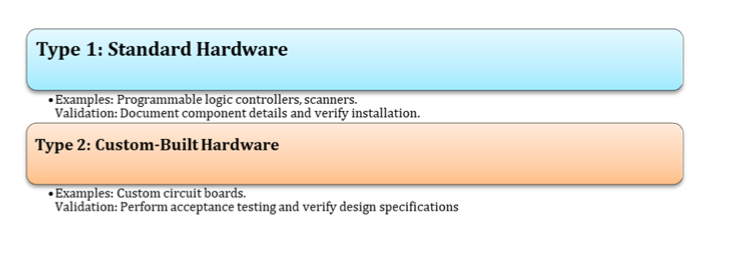
Hardware Categories
The CSV Process: A Step-by-Step Approach
CSV follows a structured life cycle aligned with the System Development Life Cycle (SDLC). The V-model, widely used in Computer System Validation, ensures thorough testing and documentation at each stage. Here’s an overview of the key phases:
- Validation Master Plan (VMP) The VMP outlines the scope, approach, and resources for validation. It covers hardware, software, and risk management, ensuring alignment with GMP and regulatory requirements.
- User Requirement Specification (URS)
The URS defines what the system must do, with each requirement uniquely identified (e.g., URS-01). This document guides system design and ensures all functionalities are tested. - Design Qualification (DQ)
DQ verifies that the system design aligns with GMP and user requirements. It involves reviewing design specifications and ensuring they meet functional and regulatory standards. - Installation Qualification (IQ)
IQ confirms that the system is installed correctly in the intended environment. It includes verifying hardware and software configurations against the installation guide. - Operational Qualification (OQ)
OQ tests the system’s functionality under static and dynamic conditions, ensuring it meets operational specifications in the user’s environment. - Performance Qualification (PQ)
PQ verifies that the system performs consistently under real-world conditions, including expected workloads and stress scenarios. - Validation Summary Report
This report compiles the results of IQ, OQ, and PQ, documenting compliance and system readiness. - Periodic Review
Regular assessments ensure ongoing compliance and system performance. Annual reviews, change control, and internal audits help maintain validation status.
Common Challenges and How to Address Them
The FDA frequently identifies CSV-related deficiencies during inspections. Here are common issues and solutions:
- Incomplete Documentation
Ensure all validation steps are thoroughly documented, including test results, deviations, and corrective actions. Maintain clear records to demonstrate compliance. - Lack of a Documented Process
Develop detailed validation procedures that define deliverables, stakeholder roles, and methodologies. A clear process reduces ambiguity and ensures consistency. - Deviations from the Validation Plan
Document any deviations, provide justifications, and implement corrective actions. Validate changes before system release to maintain compliance.
Best Practices for CSV in 2025
To achieve robust Computer System Validation and maintain compliance, consider these best practices:
- Adopt a Risk-Based Approach: Use GAMP 5’s risk-based methodology to prioritize validation efforts based on system impact and criticality.
- Leverage the V-Model: Structure validation around the V-model to ensure comprehensive testing and traceability.
- Invest in Training: Equip staff with the knowledge to implement and maintain Computer System Validation processes effectively.
- Stay Updated on Regulations: Monitor changes in FDA, EU, and ISO guidelines to ensure ongoing compliance.
- Enhance Cybersecurity: Incorporate cybersecurity measures into CSV to protect data integrity and system reliability.
- Conduct Regular Reviews: Perform periodic audits and reviews to maintain validation status and address emerging risks.
Are you interested in testing or managing data that are reliable and compliant? Contact Zenovel expert team on bd@zenovel.com
recommended: Pharmacokinetics Simplified: From Data Collection to PK Parameter Interpretation