Welcome to the Zenovel Blog!
We are a leading provider of drug regulatory consulting and education, focusing on advancing pharmaceutical innovation while ensuring safety and compliance. This blog delves into the essentials of Drug Regulatory Affairs (DRA), covering its scope, career opportunities, and intricate processes involved in bringing new drugs to market. This guide is beneficial for students, seasoned pharma experts, and industry enthusiasts.
What is Drug Regulatory Affairs?
Drug Regulatory Affairs (DRA) is the regulatory body responsible for ensuring the safety, effectiveness, and legal compliance of medicines before they enter the market. It works with global regulatory bodies to secure approvals, monitor compliance, and handle post-market surveillance, protecting public health. DRA acts as a gatekeeper, ensuring that innovative drugs adhere to standards set by agencies like the FDA, EMA, and CDSCO, from early drug development to global distribution, thereby preventing unnecessary risks in the pharmaceutical industry.
The Scope of Drug Regulatory Affairs
DRA covers a broad spectrum of activities in the pharma lifecycle. Here’s a breakdown of the key areas:
- Drug Development & Approval: Regulatory affairs in pharma involves overseeing the drug journey from lab to market, preparing and submitting applications for new drugs to ensure safety and efficacy, and maintaining high standards throughout a product’s life, addressing the question of what regulatory affairs in pharma entail.
- Regulatory Compliance & Documentation: Pharma companies must adhere to national and international regulations, including clinical trial applications, manufacturing guidelines, and safety reports, to ensure compliance with regulatory bodies.
- Quality Control & Good Manufacturing Practices (GMP): Ensuring drugs are produce consistently and safely is crucial. In regions like India, DRA focuses on CDSCO guidelines to uphold product quality.
- Pharmacovigilance & Post-Marketing Surveillance: Monitoring continues after the drug is market. This involves identifying adverse drug reactions (ADRs) and reporting problems to keep patients safe.
- Global Regulatory Affairs: Experts in international pharma must understand ICH guidelines and WHO standards, and a solid drug regulatory course can equip them with knowledge to handle country-specific processes.
At Zenovel, we emphasize how these areas interconnect to foster innovation without compromising safety.
Career Opportunities in Drug Regulatory Affairs
DRA is a thriving field with diverse roles for all experience levels. The demand for skilled professionals is on the rise as regulations evolve. Here’s a look at the career ladder:
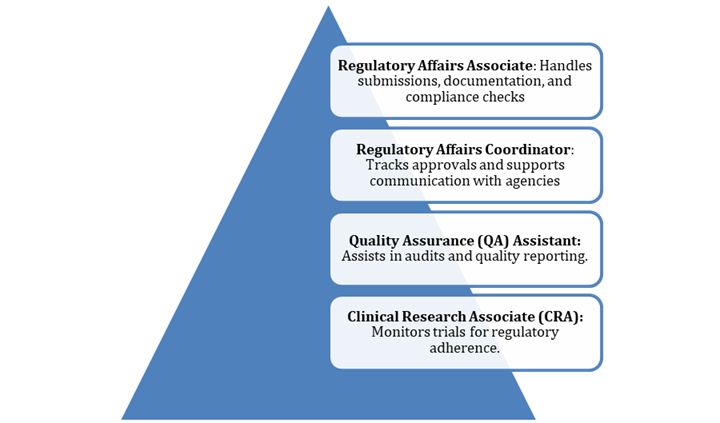
Entry-Level Roles
.
Qualifications: A bachelor’s in pharmacy, life sciences, or related fields, plus knowledge of FDA, EMA, CDSCO, and ICH guidelines.
Mid-Level Positions
- -Regulatory Affairs Specialist: Manages applications and adapts to changing regs.
- Drug Safety Officer / Pharmacovigilance Specialist: Monitors ADRs and risk strategies.
- Regulatory Compliance Specialist: Prepares for audits and ensures GMP/GCP compliance.
- Medical Writer: Crafts regulatory documents like NDAs and trial reports.
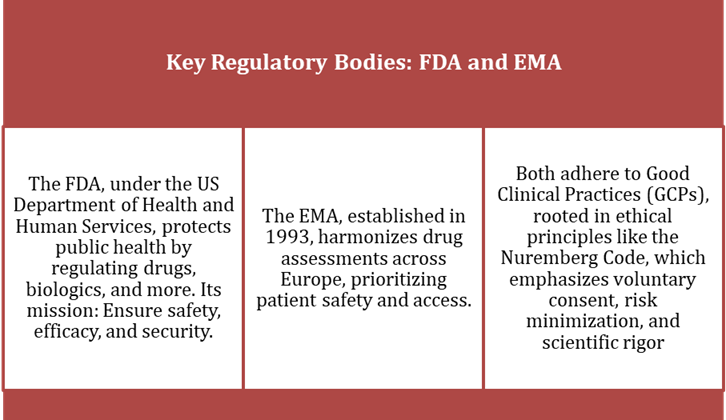
Ethical Foundations and Data for Clinical Trials
Modern drug trials are built on ethics, drawing from the Nuremberg Code’s 10 principles, such as voluntary consent and avoiding unnecessary harm. GCPs, detailed in ICH E6(R2), guide global standards.
To start human trials, sponsors submit data on:
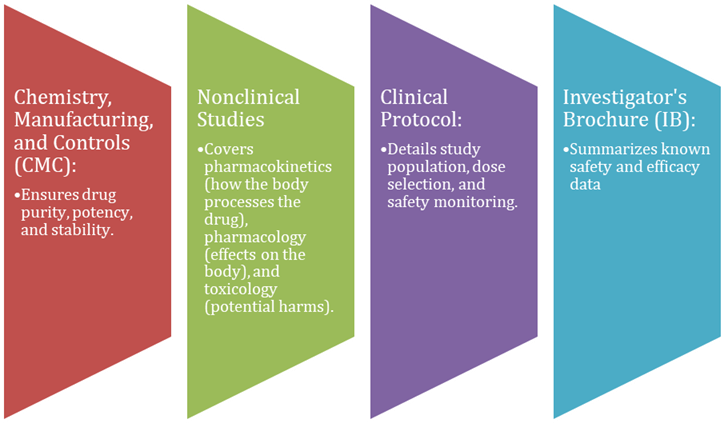
- These minimize risks in first-in-human (FIH) studies.
IND vs. CTA: Navigating Approvals
- In the US, an Investigational New Drug (IND) application clears the path for trials. It’s “cleared” in 30 days, allowing multiple studies under one IND.
- In the EU, a Clinical Trial Application (CTA) is needed per study. The upcoming CTR 536/2014 will streamline this with harmonized reviews.
Maintenance includes amendments, safety reporting, and annual updates. Differences: INDs can lead to holds; CTAs are approve or reject outright.
DRA is crucial for safe pharma innovation, and professionals who stay ahead will thrive. Zenovel offers expert guidance, courses, and solutions to excel in this field.
Explore our drug regulatory courses or contact us for consulting. Stay tuned for more insights.