
Navigating the world of pharmaceuticals, medical devices, and biotechnology is never straightforward. With the diversity of regulatory compliance governs the Code of Federal Regulations (CFR) – a regulatory path that shapes companies’ efforts and implements how companies long for their products to be developed, manufactured, and marketed. For companies trying to achieve regulated compliance, the CFR has to be part of your vocabulary. At Zenovel, we help companies think strategically about the maze of regulations to ensure smooth regulatory compliance and placement in the market. In this blog, we will discuss about the CFR, its importance to regulatory affairs and how Zenovel can help support your journey.
What is the Code of Federal Regulations (CFR)?
The Code of Federal Regulations (CFR) is an extensive collection of federal regulations developed and issued by U.S. federal agencies and codified and published in the CFR by the Office of the Federal Register. The CFR consists of 50 titles although title 21 is the title that governs the pharmaceutical industry and medical device industries because it contains the regulations that the Food and Drug Administration (FDA) enforces. Title 21 includes many aspects, such as:
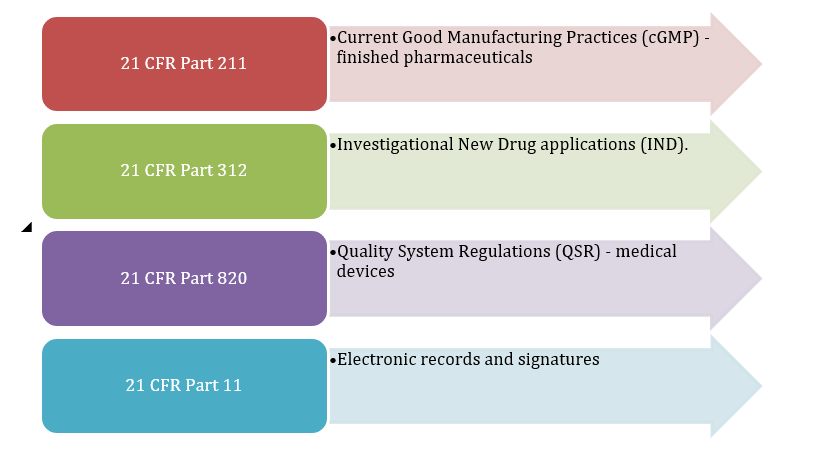
Figure 1: 21 CFR
These CFR regulations ensure regulated products are safe and effective, contributed to standardization and compliance with the federal government, and assured public health while advancing innovation.
What makes the CFR significant?
The CFR comprises the foundation for regulatory affairs, creates regulations that are binding commitments for companies to:
- Ensure Product Safety and Efficacy: Regulations like 21 CFR Part 314 set requirements for New Drug Applications (NDA), indicating drugs must meet defined safety and efficacy requirements before the market can intake it.
- Standardization of Manufacturing Practices: Being complaint with cGMP (21 CFR Part 211) demonstrates that the products being manufactured are consistent and of the defined quality.
- Ease of Getting Market Approval: Knowledge of the CFR, alleviates the time and cost of the submission process of regulatory approvals.
- Ensure continued compliance after market approval: The CFR describes the need for continual monitoring like adverse event reporting, to provide environments where products can remain safe.
The implications of being non-compliant with the CFR. Could lead to serious consequences between product recall(s), warning letters, fines or even criminal prosecution. For the pharmaceutical and medical device companies. Staying in compliance ensures their lines of products and services remain available to consumers.
Obstacles to Studying the CFR
The CFR is extensive and intricate in nature. It is loaded with thousands of pages of regulations that are updated on an annual basis. Regulatory affairs professionals have obstacles such as:
- Abreast with annual updates. The CFR is constantly evolving as new science is developed and/or as regulatory priorities change. Thus requiring vigilance by regulatory affairs professionals.
- Interpreting needs. The technical language and cross-references in the CFR may require considerable interpretation by those. Who are not experts in its application.
- Global harmonization. If companies operate internationally, compliance with the CFR must part of an overall, integrated approach with other global standards. Including the European Medicines Agency (EMA) or International Council on Harmonisation (ICH).
Zenovel’s regulatory affairs experts know how to simplify the complexities of the CFR. So, that your organization is compliant at all points in the product lifecycle.
How Zenovel Helps You Master CFR Compliance
At Zenovel, we understand that navigating the CFR can be daunting. Our comprehensive regulatory affairs solutions are design to help you achieve compliance with ease and efficiency. Here’s how we support you:
- Regulatory Strategy Development:
We craft tailored strategies to align your product development and submission processes with CFR requirements, ensuring a smooth path to market approval. - cGMP Compliance Support:
Our experts guide you through 21 CFR Part 211 compliance, helping you implement robust quality systems and avoid manufacturing pitfalls. - Submission Preparation:
From INDs to NDAs and Premarket Approvals (PMAs), we ensure your submissions are comprehensive, accurate, and CFR-compliant. - Training and Education:
We provide training on key CFR regulations, empowering your team to stay informed and proactive in maintaining compliance. - Post-Market Surveillance:
Zenovel helps you establish systems for adverse event reporting and post-market monitoring, ensuring ongoing compliance with CFR mandates.
Why Choose Zenovel?
Zenovel is a trusted partner in regulatory affairs, offering a client-centric approach and deep expertise in FDA regulations. Our goal is to ensure products meet safety and quality standards. Whether a start-up developing drug or an established company launching a new medical device, Zenovel provides. The necessary tools and guidance to succeed. The Code of Federal Regulations (CFR) is the foundation of regulatory compliance in the pharmaceutical and medical device industries. Zenovel simplifies compliance, making it efficient and stress-free.
read also: How Contract Research Organizations (CROs) Accelerate Modern Drug Development