Lipinski’s Rule of 5 (Ro5) is a crucial guideline in pharmaceutical development, helping researchers evaluate a compound’s likelihood of becoming an effective oral drug. However, with the rapid evolution of therapeutic strategies, it’s worth considering the relevance of Ro5 in the current drug design landscape.
Understanding Lipinski’s Rule of 5
The Rule of 5 is a set of criteria introduced by Christopher Lipinski in 1997 to predict a compound’s physicochemical properties for oral bioavailability, derived from the fact that each parameter involves a multiple of five.
- Molecular weight < 500 Daltons
- LogP (partition coefficient) < 5
- Hydrogen bond donors ≤ 5
- Hydrogen bond acceptors ≤ 10
A compound that fails to meet certain criteria, such as a LogP value exceeding 5, may exhibit poor absorption or limited membrane permeability, leading to poor solubility, unpredictable absorption, and potential accumulation in fatty tissues, compromising efficacy and safety.
Why LogP Is Central to Drug Design
LogP, a measure of lipophilicity, is a crucial Ro5 parameter that influences drug behaviour by determining how a drug partitions between lipid and aqueous environments.
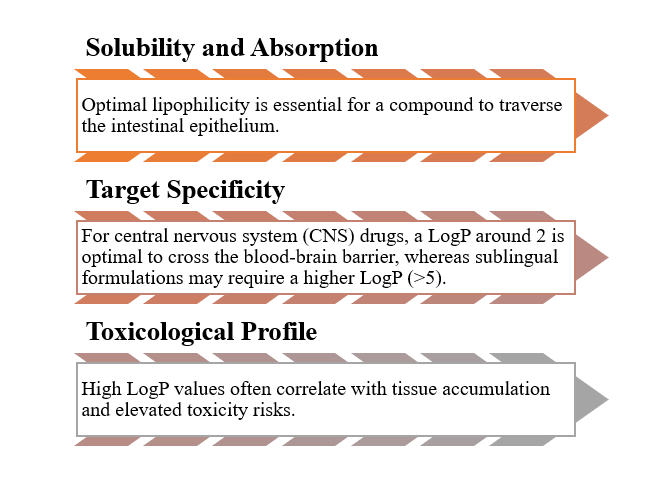
As a result, LogP does more than indicate oral bioavailability—it fundamentally shapes formulation strategies and dosing regimens.
Rule of 5 in the Era of Complex Modalities
- Lipinski’s Rule of 5 is a valuable tool in drug discovery, but its limitations are evident in modern drug modalities. Emerging drug classes, such as PROTACs, macrocyclic peptides, and covalent inhibitors, often exceed the 500 Da molecular weight limit and target previously “undruggable” targets like transcription factors and protein-protein interactions, leading to the concept of “beyond Rule of 5” (bRo5).
- Oral drug development still demands careful modulation of LogP, typically aiming for values between 1.35 and 1.8.
- Overly lipophilic compounds may suffer from poor plasma exposure and elevated toxicity—issues that Ro5 helps flag early.
New Tools for the bRo5 Generation
Traditional prediction models, trained on small, Ro5-compliant molecules, have been outperformed by the complexity of bRo5 compounds, leading to the development of modern cheminformatics tools.
- Zenovel supports customizable drug-likeness filters, allowing researchers to:
- Adjust LogP and other thresholds tailored to PROTACs and similar molecules.
- Prioritize compounds with the most favorable ADME/Tox
- pKa prediction platforms accommodate multifunctional compounds by:
- Applying localized ionization modeling.
- Leveraging extensive industry datasets (>2,500 experimental pKa values).
These tools allow scientists to re-define “lead-like” characteristics for non-traditional drugs—minimizing Ro5 violations without compromising therapeutic potential.
Looking Ahead: Intelligent Rule-Breaking with Hybrid Approaches
Future therapeutics are increasingly hybrid in nature, combining the specificity of biologics with the pharmacological versatility of small molecules. This includes:
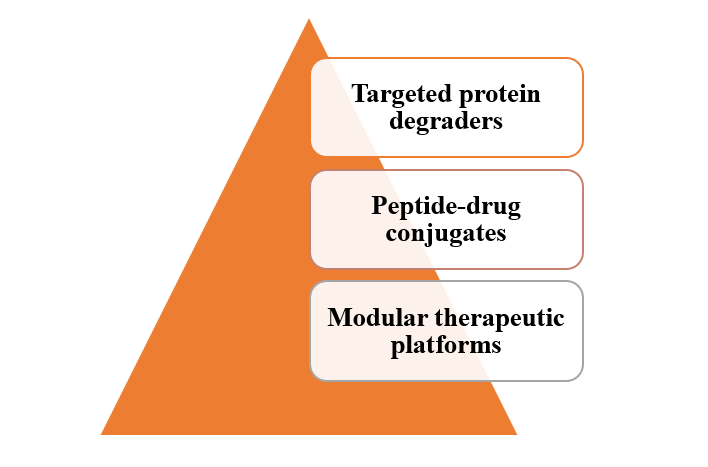
Figure 1: Hybrid Approach
AI-assisted Structure Design Engines™ are crucial for efficient molecule design, enabling early-stage optimization of key physicochemical properties and strategic balancing of conflicting factors like solubility vs. permeability.
AI-assisted Structure Design Engines™ are crucial for designing molecules effectively, enabling early-stage optimization of key physicochemical properties and strategic balancing of conflicting factors like solubility vs. permeability. Lipinski’s Rule of 5 remains a foundational tool in medicinal chemistry, offering valuable insights for developing orally bioavailable drugs. In today’s complex modalities and therapeutic innovation landscape, the rule serves best as a flexible launchpad. By pairing Ro5 with modern predictive technologies and AI-driven design, researchers can intelligently navigate its boundaries to unlock treatments for previously untreatable diseases.
Zenovel supports this evolving drug discovery process by offering advanced ADME/Tox screening, predictive modelling, and formulation expertise tailored for both Ro5-compliant and bRo5 compounds. By integrating cutting-edge tools and scientific insights, Zenovel helps accelerate lead optimization and improve oral bioavailability outcomes.
read also: The Crucial Role of Regulatory Affairs in the Pharmaceutical Industry