
Future-Ready GxP Services: How Pharma Leaders Can Stay Compliant in 2026 and Beyond
The pharmaceutical industry is facing a convergence of innovation and regulation as it approaches 2026, with GxP services playing a
We Embody a Legacy of Incredible Discoveries, Exploring the Boundaries of Innovation and Science

At Zenovel, we integrate our extensive expertise across a wide range of domains, from Good Clinical Practice to Regulatory Affairs, to present valuable insights and thought leadership. Each blog reflects our intense dedication to innovation, offering a forum to share our perspectives, groundbreaking ideas, and industry expertise. Through our blogs, we hope to uplift, inform, and support scientific, medical, and pharmaceutical innovation.

The pharmaceutical industry is facing a convergence of innovation and regulation as it approaches 2026, with GxP services playing a

The recent update of ICH E6(R3) marks a significant evolution in clinical trial execution, transitioning from a compliance-centric approach to
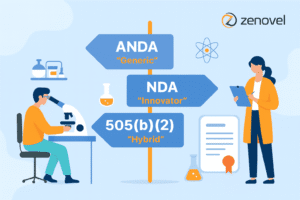
In the pharmaceutical industry, particularly in India’s expanding market, the regulatory pathway is a crucial determinant that influences the transition

In the current pharmaceutical environment, the integrity of a company’s supply chain relies heavily on its weakest link, particularly concerning
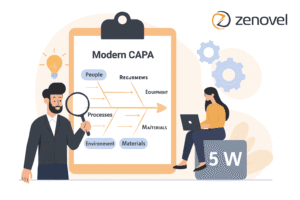
In clinical research, Good Clinical Practice (GCP) sets ethical and quality standards. Non-compliance during audits poses systemic risks to patient

The pharmaceutical industry relies on innovation, but not all companies can fully develop a drug. Strategic licensing, including In-Licensing and

The pharmaceutical industry is growing and changing rapidly. As a result of this emergence, getting a new drug to the market can be a thorny and long path from the moment of research and development to the point of regulatory approval. At every step in that journey, carefulness, compliance, and

In the ever-changing and competitive pharmaceutical and biotechnology industry, long-lasting success requires strategic foresight, flexibility, and a firm grasp of the market environment. At Zenovel, we specialize in offering expert advisory services and integrated service solutions to help organizations navigate complex environments and achieve sustained services provided to clients. Below

Clinical trials are the backbone of pharmaceutical innovation; ensuring new treatments are safe and effective. However, managing these trials is no small feat—complex data, regulatory compliance, and tight timelines demand robust solutions. Clinical Trial Management System (CTMS), specialized software designed to streamline trial processes, enhance data accuracy, and improve operational

Moving forward into 2026, the pharmaceutical and healthcare industries are embarking on a transformation with artificial intelligence (AI) as a catalyst for change in regulatory affairs. At Zenovel, we see great potential for AI technology to be integrated into compliance efforts to create efficiencies, accuracy, and strategic choices. This blog

In today’s era, Zenovel is enhancing Pharmacovigilance, the monitoring and evaluation of pharmaceutical products, by promoting international collaboration, utilizing advanced technologies, and integrating local expertise, thereby ensuring safer medications for diverse populations worldwide. The Power of International Collaboration Pharmacovigilance Zenovel is forming interconnected pharmacovigilance networks, enabling countries to share critical

Pharmacovigilance is crucial for public health, ensuring the safety and effectiveness of medicinal products throughout their lifecycle. It involves periodic evaluation of real-world data to monitor a drug’s safety profile post-market release. Zenovel aims to discuss the evolution of Periodic Safety Update Reports into Periodic Benefit-Risk Evaluation Reports. Why Ongoing
We provide affordable, innovative, and high-quality solutions to pharma industry with ethics driven research and increasing accessibility to high quality medicine worldwide
Copyright © 2024 Zenovel. All rights reserved.