Pharmacokinetics (PK) is a key element of drug development, and describes how a drug interacts with the body by defining its absorption, distribution, metabolism and excretion (ADME). Knowing about PK is essential in drug development to establish the safety and efficacy of drugs (both new and generic). It is Zenovel’s responsibility to provide a simplified overview of the complex world of PK analysis, taking you from data collection to understanding the main Pharmacokinetics parameters in the context of Bioavailability (BA) and Bioequivalence (BE).
What is Pharmacokinetics?
Derived from the Greek words pharmakon (drug) and kinetikos (position in motion), pharmacokinetics describes what the body does to a drug after administration. It’s a vital assessment in drug development, helping researchers determine safe and effective dosing levels. PK analysis tracks how a drug’s concentration changes over time, providing a window into its therapeutic potential and how the body processes it.
In BA/BE research, PK analysis compares the bioavailability of a test drug to a reference drug to ensure therapeutic equivalence. This involves studying parameters like peak concentration (Cmax), total exposure (AUC), and the time to reach peak concentration (Tmax). These metrics help confirm that a drug delivers. The same therapeutic benefits as its reference, a critical step for regulatory approval.
The PK Analysis Process: From Data to Insights
PK analysis involves collecting blood samples after drug administration. To track plasma concentration, revealing drug absorption, distribution, metabolism, and elimination, using two primary approaches for data analysis
- Non-Compartmental Analysis (NCA): NCA is a popular method for estimating PK parameters like AUC, Cmax, and Tmax without relying on specific drug distribution models or in vivo processes.
- Compartmental Modeling: The approach involves modeling drug behavior in detail by assuming the body has compartments like central and peripheral areas, which is beneficial for complex concentration-time profiles.
By examining key parameters, PK analysis provides critical insights into a drug’s bioavailability and therapeutic effects.
Key PK Parameters Explained
Understanding PK parameters is essential for interpreting a drug’s behavior. Here are the five most important parameters in BA/BE studies:
- Bioavailability (F): The drug’s bioavailability is influenced by the absorbed fraction (fa) and the fraction escaping liver metabolism (fh), with a high first-pass effect reducing bioavailability and potentially affecting its effectiveness.
- Volume of Distribution (Vd): The distribution phase of a drug, from plasma to tissues, influences its speed of action, indicating its widespread spread after a loading dose.
- Clearance (CL): The rate at which a drug is removed from the body, either renal or non-renal, is typically dose-independent, meaning changes in dose result in proportional changes in plasma concentration.
- Half-Life (t1/2): Half-life refers to the time it takes for a drug to decrease by 50%, reach steady-state concentrations with repeated dosing, and eliminate the drug after stopping treatment.
- Protein Binding: Pharmacological activity of a drug depends on its unbound fraction, and changes in protein binding, like renal or hepatic impairment, can significantly affect drug exposure and therapeutic effects.
Challenges in PK Analysis
While Pharmacokinetics analysis is powerful, it comes with challenges:
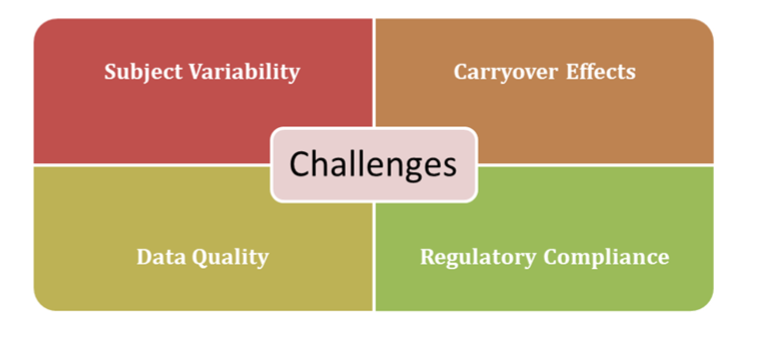
- Subject Variability: Individual differences in metabolism and absorption can lead to variability in PK parameters, complicating comparisons.
- Carryover Effects: In crossover studies, inadequate washout periods between doses can bias results if the first drug lingers in the system.
- Data Quality: Missing or inaccurate measurements can skew conclusions, emphasizing the need for rigorous data collection.
- Regulatory Compliance: Adhering to diverse guidelines (e.g., FDA, EMA) requires constant updates to study protocols.
Statistical Methods in BA/BE Studies
Statistical analysis ensures that differences in PK parameters between test and reference drugs are within acceptable limits. Key methods include:
- ANOVA (Analysis of Variance): Compares means of PK parameters like AUC and Cmax, accounting for variability within and between subjects.
- Confidence Interval (CI) Approach: Bioequivalence is confirmed if the 90% CI for the ratio of AUC and Cmax falls within 80%–125%.
- Mixed-Effects Models: Handle complex data structures by accounting for fixed and random effects.
- Bootstrapping: A resampling technique used when data don’t meet traditional statistical assumptions.
- Power Analysis: Determines the sample size needed to detect significant differences, ensuring robust study design.
Advances in PK Analysis
Advancements in PK data generation have led to more accurate and less variable parameters. Cannulated animal studies yielding more accurate data. Cassette dosing accelerates screening by providing up to ten times more data. Than, traditional studies, and bile excretion studies provide insights into drug elimination pathways.
Why Pharmacokinetics Matters in Drug Development
PK analysis is crucial for drug safety and efficacy in preclinical and clinical studies. It helps confirm therapeutic equivalence, optimize dosing, and meet regulatory standards. As analytical tools and guidelines evolve, PK analysis drives faster, more accurate drug assessments, benefiting patients and healthcare systems worldwide.
Get Zenovel’s Expert Guidance on PK Analysis
Have questions about Pharmacokinetics or statistical methods in BA/BE research? Contact our expert team on bd@zenovel.com for clear explanations and tailored guidance to support your study’s success. Whether you’re navigating complex PK parameters or ensuring regulatory compliance, we’re here to help.
read also: The Evolution of Clinical Trial Monitoring: From On-Site to Remote Risk-Based Approaches