
QMS setup plays a crucial role in the pharmaceutical industry, as the foundation of patient safety and product efficacy lies in a consistent Quality Management System (QMS). This system underpins Good Manufacturing Practices (GMP), guaranteeing that each drug produced adheres to rigorous standards regarding safety, purity, and performance. The blog elaborates on the components and significance of a QMS within GMP and highlights how Zenovel’s expert QMS setup services can assist organizations in establishing or improving their QMS.
What Is a Quality Management System in GMP?
A QMS is a structured framework comprising policies, processes, and procedures aimed at overseeing and regulating an organization’s quality-related activities. In the pharmaceutical sector, it is aligned with current Good Manufacturing Practices (cGMP) as mandated by regulatory authorities such as the FDA, EMA, and WHO. As part of an effective QMS setup, the International Council for Harmonisation (ICH) Q10 guideline outlines a detailed model for a Pharmaceutical Quality System (PQS), which reinforces GMP obligations throughout the product lifecycle—from development and technology transfer to manufacturing and discontinuation. This guideline focuses on science-based, risk-informed strategies, promotes continual improvement, and emphasizes the importance of knowledge management.
Robust QMS go beyond basic compliance checklists by embedding quality into every process to prevent issues such as contamination, mix-ups, or deviations. An effective QMS setup consists of several interconnected elements essential for GMP compliance.
Quality Manual and Policies: Outlining objectives, organizational structure, and quality commitments.
Document and Data Management: Controlled procedures, records, and SOPs for traceability.
Process Performance Monitoring: Ongoing evaluation of manufacturing processes.
Corrective and Preventive Actions (CAPA): Systems to address deviations and prevent recurrence.
Change Management: Controlled modifications to processes, equipment, or materials.
Deviation Handling: Documentation and investigation of non-conformances.
Risk Management: Proactive identification and mitigation of risks (per ICH Q9).
Management Review: Regular oversight to drive continual improvement.
Internal Processes and Client Satisfaction: Focus on customer needs and product quality evaluation.
These components align with ICH Q10’s four primary elements: monitoring, CAPA, change management, and management review.
Why a Strong QMS Matters for GMP Compliance
GMP regulations minimize risks that cannot be detected through final product testing alone, such as cross-contamination or labeling errors. A well-structured QMS setup ensures that processes are controlled, documented, and continuously improved to safeguard product quality and patient safety.

Implementing a QMS setup requires conducting a gap analysis, customizing it to fit organizational needs, training personnel, and validating the system. Continuous observation should be encouraged for ongoing improvements in processes, materials, and documentation, while effective deviation systems are essential for documenting and promptly resolving issues.
Zenovel Supports QMS Setup for GMP
Zenovel specializes in GMP-compliant QMS setup, providing customized solutions that enhance performance and ensure compliance with regulations.
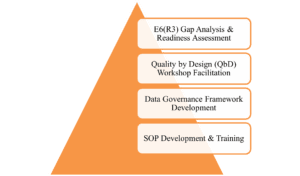
Figure 1: Zenovel QMS Setup for GMP
Zenovel offers innovative services for pharmaceutical companies, focusing on ethical practices and high-quality medicine. They help establish new QMS setup frameworks and optimize existing systems, ensuring compliance and operational excellence. By adopting modern frameworks like ICH Q10, pharmaceutical companies can achieve reliable, high-quality production, protecting patients and driving business growth.
For more details, visit https://zenovel.com/gmp/quality-management-system-set-up/