
Clinical trials are crucial for drug development, but managing them is complex due to the vast amount of data and patient safety concerns. Zenovel uses innovative approaches like Risk-Based Monitoring (RBM) to streamline processes, prioritize quality, and enhance trial efficiency. This blog explores RBM’s significance in clinical trials and how Zenovel leverages it.
What is Risk-Based Monitoring (RBM)?
Risk-Based Monitoring (RBM) is a strategic clinical trial monitoring method that uses advanced analytics and technology to identify, assess, and mitigate risks that could affect trial quality, patient safety, or data integrity, thereby ensuring proactive risk management and optimizing trial efficiency. RBM was developed in response to FDA and EMA guidance, promoting risk-based approaches for efficient clinical trial monitoring. The International Council for Harmonisation’s E6 (R2) guidelines in 2016 further solidified RBM’s role by requiring sponsors to develop risk-based methods for assessing and managing trial risks.
How RBM Works in Clinical Trials
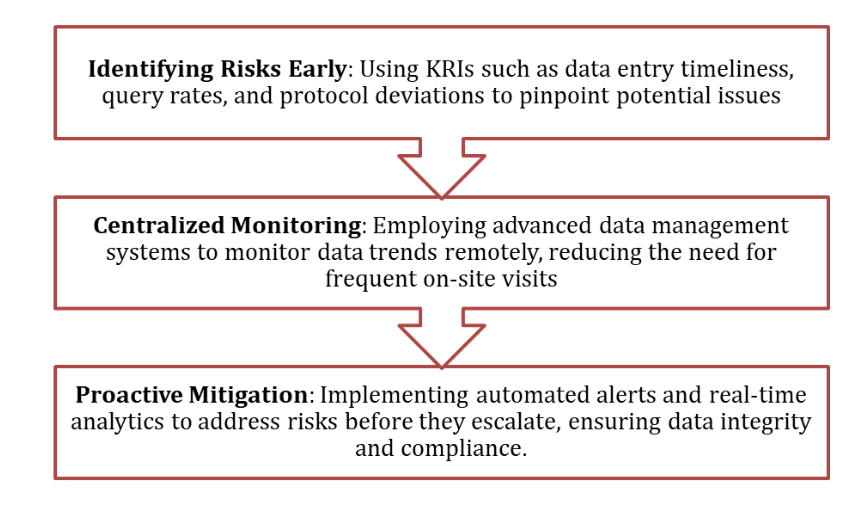
RBM is a method that identifies critical data and processes that could impact trial outcomes. It uses tools like Key Risk Indicators (KRIs) and advanced analytics to monitor potential risks, such as delayed data entry, high query rates, protocol deviations, or missing data, which could compromise trial results or patient safety.
At Zenovel, we integrate RBM into our clinical trial processes by:
Benefits of RBM in Clinical Trials
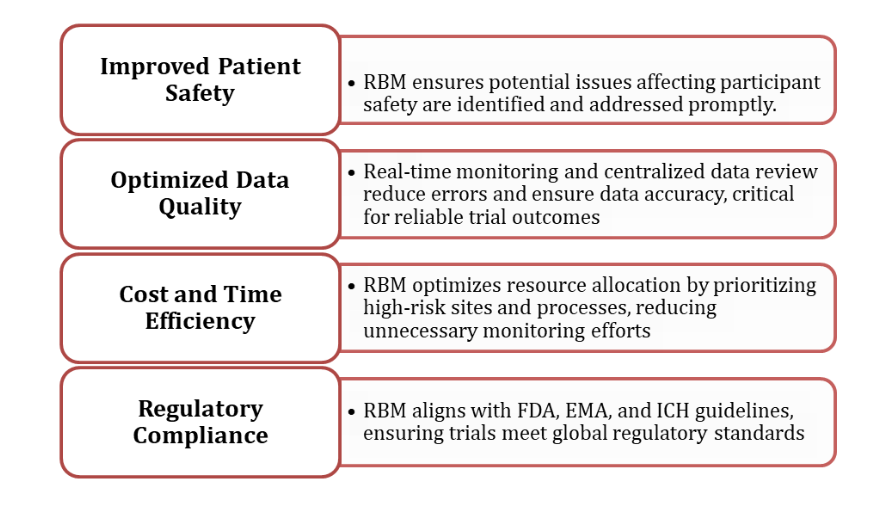
RBM offers several advantages that align with Zenovel’s commitment to delivering high-quality, efficient clinical trials:
RBM vs. Risk-Based Quality Management (RBQM)
RBM is a crucial part of Risk-Based Quality Management (RBQM), a framework that covers the entire trial lifecycle from protocol design to monitoring. It involves risk assessment, control, and continuous monitoring to ensure quality at every stage. Zenovel views RBM as a key pillar in their RBQM strategy, ensuring robust protocols and trial integrity.
The Role of Technology in RBM
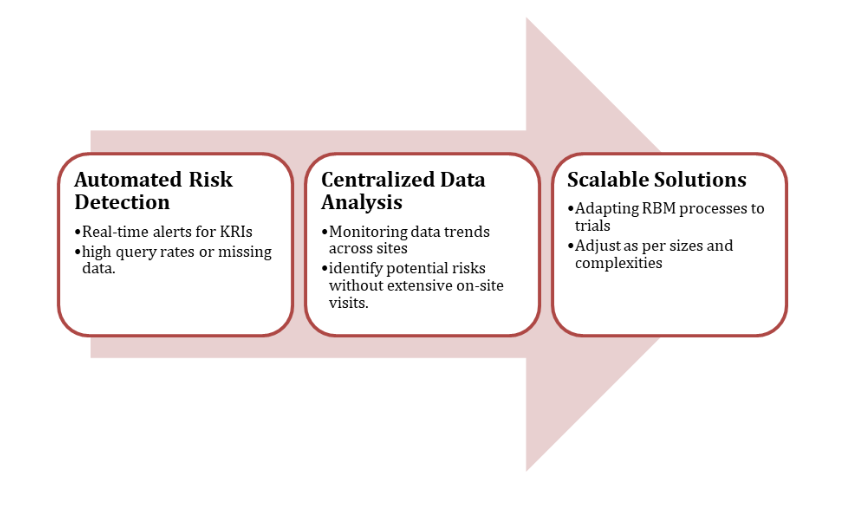
Zenovel utilizes digital tools and decentralized trial models to enhance clinical trial management, utilizing EDC systems and CTMS for the efficient implementation of Randomized Block Trials (RBB).
These tools enable
Zenovel’s Approach to RBM
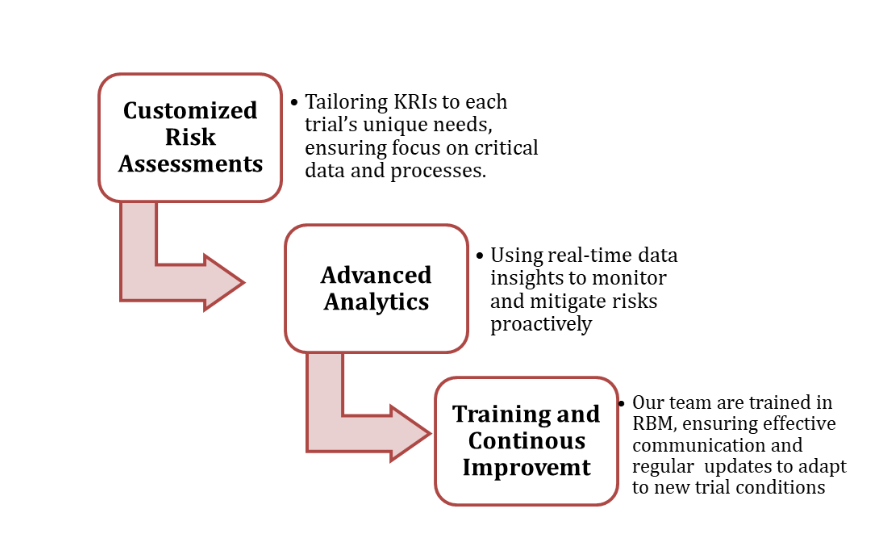
At Zenovel, RBM is more than a regulatory requirement—it’s a commitment to excellence in clinical research. Our approach includes:
Hence, Risk-Based Monitoring is transforming clinical trials by enabling smarter, more efficient, and safer research processes. At Zenovel, we are proud to lead the way in adopting RBM as part of our broader RBQM strategy. By leveraging advanced technology, regulatory insights, and a proactive approach to risk management, we ensure that our trials deliver reliable results while prioritizing patient safety and data integrity. As the clinical research landscape continues to evolve, Zenovel remains committed to harnessing RBM to drive innovation, streamline operations, and deliver high-quality outcomes for our clients and participants.
recommended: GMP Computer System Validation (CSV): A Complete Guide for Pharma Compliance in 2025