Regulatory affairs (RA) play a crucial role in the pharmaceutical industry; ensuring products meet quality, safety, and efficacy. As the industry grow further the need for qualified professionals to bridge gaps between companies and regulators increases. This blog explores Zenovel regulatory affairs expertise, our various facets, and valuable contribution to the pharmaceutical and biotechnology industry.
What is Regulatory Affairs?
Regulatory affair is a specialized profession that acts as the interface between pharmaceutical companies and regulatory bodies worldwide, such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and India’s Central Drugs Standard Control Organization (CDSCO). RA professionals ensure healthcare products, medical devices, biologics, and cosmetics meet international and regional standards before marketing, safeguarding public health and enabling companies to efficiently introduce innovative products to the market.
The Progression of Regulatory Affairs
Modern regulatory affairs originated in the 1950s due to pharmaceutical tragedies resulting from manufacturing errors or intentional adulteration. Global regulatory bodies established guidelines like GMP and MA protocols to ensure product safety, quality, and efficacy. Over time, the Regulatory Authorization (RA) profession has evolved to address drug development complexity and the dynamic regulatory environment, making it a crucial part of the pharmaceutical industry.
Significant Roles of Zenovel Regulatory Affairs Professionals
Regulatory affairs professionals play a pivotal role across the entire lifecycle of a pharmaceutical product, from development to post-marketing activities. Their responsibilities include:
- Liaison with Regulatory Agencies: RA professionals serve as the primary liaison between companies and regulatory authorities, ensuring clear communication and adherence to regulations.
- Document Preparation and Submission: Our RA professionals manage and review critical documents like dossiers to meet regulatory submission deadlines, adhering to guidelines like CGMP, ICH, GCP, and GLP, ensuring compliance with Good Manufacturing Practices.
- Compliance and Audits: Zenovel’s RA team manage compliance with regulatory requirements, review audit reports, and facilitate inspections by regulatory bodies or customers.
- Pharmacovigilance: We at Zenovel monitor the safety of drugs post-approval, ensuring adverse effects are reported and addressed promptly.
- Strategic and Technical Guidance: RA professionals assist in translating regulatory requirements into actionable plans, minimizing delays, and ensuring market readiness through their expertise in R&D, production, and quality control.
Our RA professional team ensure compliance with evolving legislation across regions, helping companies avoid potential issues like inadequate documentation, flawed scientific approaches, or poor data presentation.
Scope of Regulatory Affairs in the Pharmaceutical Industry
The scope of RA professionals work in various settings, including pharmaceuticals, medical devices, in-vitro diagnostics, biologics, biotechnology, nutritional products, cosmetics, and veterinary products:

Figure 1: Scope of Regulatory Affair
The multitasking of RA professionals makes them indispensable in ensuring that products meet both scientific and commercial objectives.
Global Regulatory Bodies
Regulatory affairs professionals must be well-versed in the guidelines of various international regulatory bodies, including:
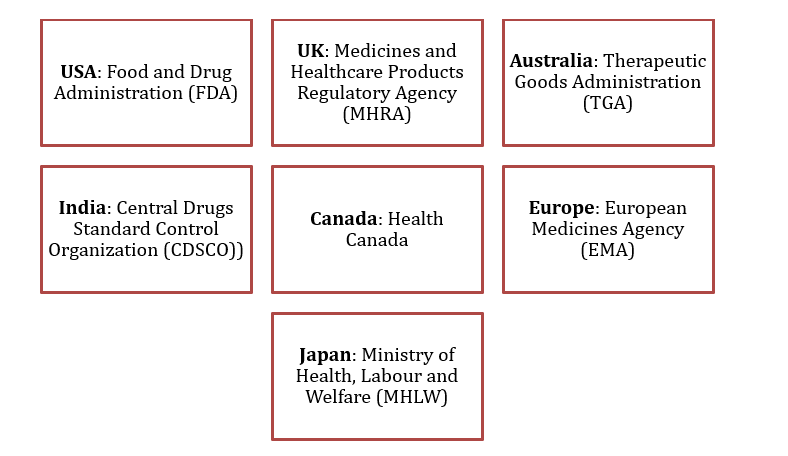
Figure 2: Global Regulatory Agencies
Each of these bodies enforces specific regulations, and RA professionals must tailor their strategies to comply with regional requirements.
The Strategic Importance of Regulatory Affairs
The development and launch of a new pharmaceutical product can take up to 10-15 years, involving scientific and regulatory challenges. RA professionals offer strategic and technical advice to R&D, production, and QC teams, ensuring product compliance.
- Decrease Time to Market: RA professionals streamline compliance processes, enabling companies to quickly bring products to market, a crucial aspect in today’s competitive market.
- Mitigating Risks: They ensure accurate documentation and regulatory adherence to prevent costly delays or rejections.
- Enhancing Safety: RA professionals uphold public health by ensuring the availability of safe and effective products through strict pharmacovigilance and compliance.
The Future of Regulatory Affairs
The regulatory affairs (RA) profession is a vital part of the pharmaceutical industry, ensuring compliance and safety. Despite economic fluctuations, the profession remains resilient. As it plays a crucial role in bridging the gap between companies and regulatory authorities. RA professionals navigate complex regulations, reduce time to market, and uphold high standards of safety and efficacy. As the pharmaceutical industry continues to innovate, the demand for skilled RA professionals will grow. Making it a rewarding and dynamic career path. For companies like Zenovel, investing in robust RA expertise is not just a compliance necessity but a strategic advantage that drives success in the global pharmaceutical landscape.
References:
- P. Praneeth, Regulatory Affairs and its Role in Pharmaceutical Industry, SSRG International Journal of Pharmacy and Biomedical Engineering, Volume 3, Issue 1, Jan-Apr 2016.
- Douglas J. Pisano and David S. Mantus, FDA Regulatory Affairs: A Guide for Prescription Drugs, Medical Devices, and Biologics, Second Edition.
- Sachan C. Utkar, Dr. Ns Vyawahare, Drug Regulatory Affairs, Third Edition (2015).
read also: Understanding the Code of Federal Regulations (CFR) in Regulatory Affairs