The international pharmaceutical landscape is inherently connected to the global economy and global trade, which is consisted with the quality and safety assurance , well-controlled quality and safety. Compliance with international pharmaceutical standards has changed from an optional step to a requirement. The Pharmaceutical Inspection Co-operation Scheme (PIC/S) is the framework for international harmonization of Good Manufacturing Processes (GMP). The scheme is currently comprised of 50+ regulatory authorities globally, all of which work to ensure a high quality of medicines is achieved so medicines can be exported and can avoid duplicative inspections. For pharmaceutical companies, compliance with PIC/S GMP is more than just checking off all items on our compliance checklist! Getting PIC/S GMP compliant is a step towards good credibility and streamlining our operations so we can access new markets. This Zenovel blog involves established knowledge regarding PIC/S to describe actionable measures for companies looking to achieve compliance more speedily and intelligently. Whether you’re a mid-sized manufacturer or a giant manufacturer, targeted improvement can help you through the route to compliance quicker and minimise disruption.
An Introduction to PIC/S
PIC/S is an informal and non-binding collusion of regulatory authorities that share information and ideas concerning GMP for human and veterinary medicines. In the 1970’s, PIC/S was created from the original Pharmaceutical Inspection Convention to effectively deal with concerns over globalization, trust and mutual recognition through the introductory ideas of developing common standards, inspector training and information exchange. PIC/S is not a treaty but allows a common and flexible framework for the authorities to work together through cooperation with no legal responsibility.
Ultimately, PIC/S promotes the development and maintenance of harmonized GMP standards, ensuring inspectorates has operating and equivalently quality systems. As of 2025, it has 56 participating authorities situated in regions extending from Europe to the Asia-Pacific region and is still growing to increase harmonization. Recent updates, guidance on conducting remote assessments in January 2025, are examples of its evolution in today’s era where remote or digital inspections are becoming the norm. For businesses, PIC/S compliance means that a GMP certification obtained from one member authority, is often accepted by other participating member authorities, therefore reducing the number of audits, and facilitating export opportunities..
The PIC/S GMP Guide: A Guide to Standards
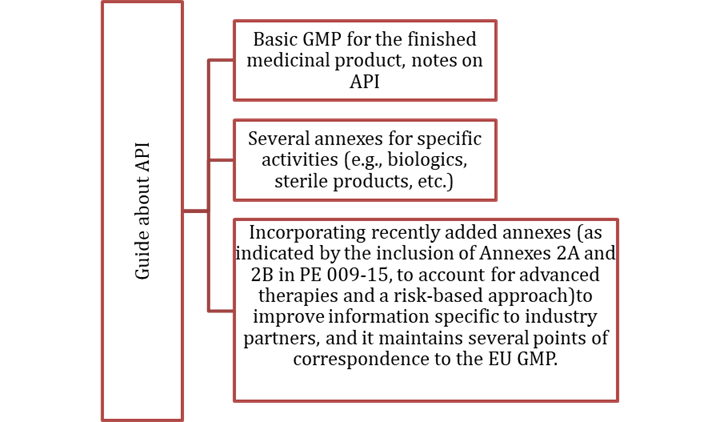
The PIC/S GMP Guide (PE 009) is the bedrock of PIC/S. The GMP Guide provides principles to guide the manufacture of medicinal products and active substances (API).

Step-by-Step: How to Achieve PIC/S GMP Compliance
Securing PIC/S-level certification involves aligning operations with guide requirements. Verified through inspections by national authority or equivalent bodies, using a structured approach.
- Start by analyzing your current state against the PIC/S GMP Guide, reviewing your quality assurance, risk management, and product review processes. Utilize tools like self-audits or third-party consultants to identify discrepancies.
- Develop a robust Quality Management System (QMS) by integrating quality assurance, control, and risk management into daily operations. Conducting regular product reviews, and thoroughly documenting all processes.
- Train and empower personnel by focusing on key roles, hygiene standards, and ongoing training, creating programs covering GMP basics and specialized topics, and fostering a compliance culture.
- Design facilities to prevent mix-ups, create dedicated areas for production. Storage, and testing, and implement digital documentation systems for better control, retention, and traceability of specs, formulas, and records.
- Enhance production and control practices by prioritizing contamination controls, material checks, and stability monitoring, and defining clear contracts for outsourced work.
- Develop efficient systems for handling complaints and recalls, practicing mock inspections, including remote ones, in accordance with the new 2025 guidelines.
- To obtain GMP certification, align with your local regulator and apply for inspection. If successful, your GMP certificate gains international weight through PIC/S networks.
PIC/S vs. WHO GMP: Key Differences to Navigate

Transitioning from WHO to PIC/S compliance might involve upgrading to these specifics. But, the overlap means it’s an evolution, not a complete overhaul.
To speed things up without overspending:
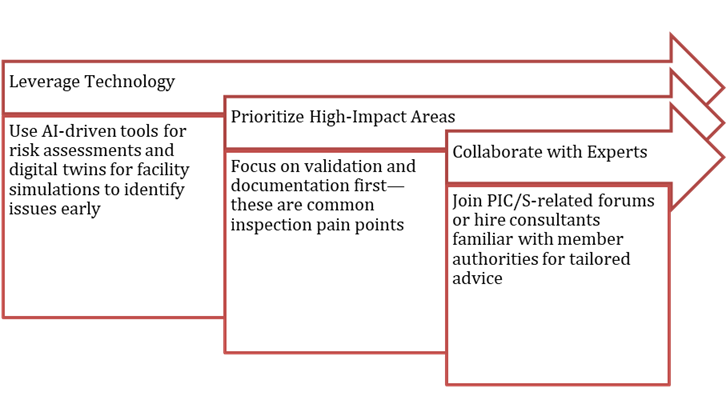
Stay Updated and Monitor PIC/S publications for revisions, like the 2025 remote inspection aids, to future-proof your systems.
Government incentives or partnerships can help smaller enterprises offset upgrade costs, transforming compliance into a competitive advantage. PIC/S alignment reduces inspection burdens, enhances market access. Builds trust with global partners, and positions a company as a quality leader. Potentially lowering costs and signaling readiness for high-stakes exports. Achieving PIC/S GMP compliance is a journey towards excellence that benefits efficiency and reputation. Addressing gaps, embracing smart tools, and staying agile are key steps. Quality that withstands global scrutiny is essential. If your authority isn’t a PIC/S member, advocate for it to benefit the entire industry. Connect with Zenovel for complete PIC/S Guide to start your journey.