Developing new medications is a complex and costly scientific process that takes 10-15 years and requires billions of dollars to transform a potential chemical into a life-saving treatment accessible to the neediest.
Zenovel works closely with pharmaceutical companies and biotechnology firms to guide them through the complex phases of drug development. We integrate scientific knowledge, regulatory expertise, and operational assistance to help innovators convert groundbreaking concepts into effective treatments. We focus on the five key phases of drug development, and explore how Zenovel contributes to success in each phase.
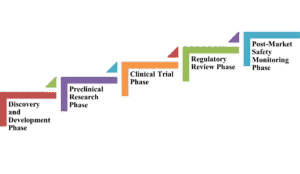
- Discovery and Development Phase
The initial phase of research focuses on identifying a promising molecule or compound for treating a disease, analyzing its biological pathways, analyzing its absorption and processing by the body, and optimizing potential candidates for evaluation.
- Zenovel’s Contributions:
Utilizing advanced data analytics tools, researchers can expedite the identification of therapeutic targets, enabling faster screening of vast compound libraries. At Zenovel, also conduct in-depth formulation assessments and feasibility analyses to determine optimal dosing regimens and delivery methods, and conduct preliminary safety and effectiveness evaluations to select promising candidates.
- Preclinical Research Phase
This stage involves in vitro and animal-based experiments to assess a drug’s safety, potential toxicity, pharmacological behavior, and formulation, adhering to Good Laboratory Practices (GLP) to meet regulatory requirements and maintain data integrity.
- Zenovel’s Contributions:
Zenovel involves in overseeing preclinical study designs, ensuring reproducibility and minimizing errors, and conducting detailed pharmacokinetic and pharmacodynamic analyses to understand the compound’s behavior. We also assists with Investigational New Drug (IND) research, compiling comprehensive data submissions to facilitate smooth transitions to human trials. For more info on Biological Services click here.
- Clinical Trial Phase
The drug undergoes four phases of human testing to confirm its safety, dosage, efficacy, and side effects, a time-consuming and costly segment with only a small percentage of candidates succeeding.
- Zenovel’s Contributions:
At Zenovel, our efficient medical writing team and clinical trial experts manages clinical trials from Phase I to Phase IV, including site selection, protocol development, and logistical coordination. We offer expert medical oversight, adverse event reporting, and pharmacovigilance services to ensure participant safety and data accuracy. We also provide advanced biostatistical analysis and data handling for informed decision-making. Further, we implement innovative strategies for patient recruitment and retention to enhance trial efficiency. More on GCP.
- Regulatory Review Phase
After successful clinical trials, a drug’s sponsor submits a detailed application, such as a New Drug Application (NDA) for small molecules or a Biologics License Application (BLA) for biologics, to agencies like the FDA, EMA, or CDSCO, which review safety, effectiveness, and production standards.
- Zenovel’s Contributions:
Our role involves assembling and filing comprehensive regulatory submissions, such as NDAs, BLAs, CTAs, and MAAs, with meticulous attention to detail. We also involves acting as a liaison with global regulatory bodies, ensuring that Chemistry, Manufacturing, and Controls documentation aligns with stringent regulatory criteria. Zenovel provides end to end Regulatory Affairs services.
- Post-Market Safety Monitoring Phase
Obtaining approval is a significant achievement, but continuous monitoring is necessary to ensure safety, detect unusual side effects, and maintain product labeling and promotional guidelines.
- Zenovel’s Contributions:
At Zenovel, we understand the importance of establishing robust pharmacovigilance frameworks for continuous patient tracking, developing risk assessment plans, and implementing advanced signal detection methods to identify potential issues early and mitigate them effectively, while adhering to evolving regulatory standards.
Overall, Zenovel provides comprehensive support throughout the drug development lifecycle, leveraging its expertise in scientific research, clinical execution, and regulatory navigation, to ensure efficient and compliant solutions that alleviate suffering and improve quality of life.
Collaborate with Zenovel to bridge the gap between your visionary research and transformative therapies that make a meaningful impact on global health.