In the pharmaceutical industry, particularly in India’s expanding market, the regulatory pathway is a crucial determinant that influences the transition from laboratory research to pharmacy shelves. With increasing demand for regulatory affairs services in India, this pathway is not merely a formality; it plays a significant strategic role, affecting factors such as time, costs, and overall success in the marketplace. Therefore, gaining a comprehensive understanding of these regulatory routes is essential for pharmaceutical companies aiming for quicker and more effective entry into the market.
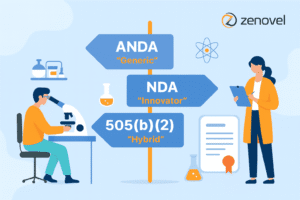
Let’s demystify the three primary pathways for drug approval: ANDA, NDA, and 505(b)(2).
-
ANDA (Abbreviated New Drug Application): The “Generic” Pathway
- What it is: An application has been made for a generic version of a drug that is already approved and recognized as the Reference Listed Drug.
- Key Requirement: You must demonstrate bioequivalence, showing that your generic drug functions the same as the original drug.
- Advantage: This is typically the fastest and least expensive route, as it avoids the need for costly clinical trials to ensure safety and efficacy.
- When to use it: When launching a generic equivalent of an off-patent drug, it is important to consider regulatory guidelines, market competition, pricing strategies, and the potential for market access barriers.
-
NDA (New Drug Application)- The “Innovator” Pathway
- What it is: A full application for a new, never-before-approved chemical entity (NCE).
- Key Requirement: Submission of comprehensive non-clinical and clinical trial data is essential to demonstrate safety and efficacy.
- Challenge: This pathway is the longest, most complex, and most expensive, requiring years of research and extensive data generation.
- When to use it: Being the original innovator of a new molecule entails a unique position in the realm of scientific discovery and intellectual property, highlighting the significance of innovation in chemistry and pharmaceuticals.
-
505(b)(2) Application– The “Hybrid” or “Smart Reformulation” Pathway
- What it is: A special type of NDA allows reliance on the FDA‘s findings regarding a drug’s safety and effectiveness for a modified product.
- Key Requirement: A special type of NDA allows reliance on the FDA’s findings regarding a drug’s safety and effectiveness for a modified product.
- Strategic Advantage: It provides market exclusivity similar to a Non-Disclosure Agreement (NDA) but is quicker and less expensive than initiating a full NDA.
When to use it: Ideal for:
- New dosage forms (e.g., tablet to oral spray).
- New strengths or routes of administration.
- New combinations of approved drugs.
- Change from prescription (Rx) to over-the-counter (OTC).
Why Expert Regulatory Affairs Services Matter in India
India’s regulatory landscape, managed by the Central Drugs Standard Control Organization (CDSCO), is intricate and requires expertise to navigate effectively, as errors can have significant consequences.
- Costly delays and re-submissions.
- Lost market opportunity and revenue.
- Failed investments in clinical trials.
Zenovel’s Regulatory Affairs Services are designed to be a crucial asset in navigating regulatory challenges in India. The company emphasizes that their role extends beyond merely filing paperwork, positioning themselves as architects of strategic pathways to market access.
How Zenovel Simplifies and Accelerates Your Journey:
- Pathway Optimization: We evaluate your product portfolio and offer strategic advice on the most efficient pathways, including ANDA, NDA, or 505(b)(2), to achieve quicker market entry and maximize return on investment (ROI).
- Comprehensive Application Management: Our team manages the complete process from dossier compilation (Modules 1-5 according to CTD requirements) to submission and communication with CDSCO.
- Bioequivalence & Clinical Trial Support: We focus on designing and managing essential studies for Abbreviated New Drug Application (ANDA) and 505(b)(2) approvals, guaranteeing adherence to both Indian and international regulatory standards.
- Post-Approval Compliance: Our support encompasses various aspects such as license renewals, accommodating post-approval changes (variations), and managing the ongoing lifecycle of the license.
- DCGI/CDSCO Expertise: Our team possesses extensive expertise in maneuvering through the intricacies of the Indian regulatory environment, facilitating transparent communication and reliable timeframes.
Zenovel offers regulatory affairs services aimed at simplifying the complexities of entering the Indian market, thereby accelerating the process of bringing products to patients. Visit us at www.zenovel.com or write to us at bd@zenove.com.