In the pharmaceutical and medical device industries, the presentation of critical information to patients has evolved from mere best practices to regulatory requirements. Regulatory User Testing (RUT), also known as Readability User Testing or Usability Testing tailored for patient information, is essential for ensuring that patients can easily comprehend important details. This blog post discusses the components of RUT, its significance in the regulatory environment, and the heightened focus from regulatory bodies such as the FDA and EMA on these testing processes. Additionally, it highlights how Zenovel, a reputable entity in regulatory affairs and drug development, assists organizations in effectively meeting these regulatory demands.
Understanding Regulatory User Testing
Regulatory User Testing is a methodical evaluation intended to gauge the clarity and usability of materials aimed at patients, such as Package Inserts (PIs), Patient Information Leaflets (PILs), Instructions for Use (IFUs), and digital health interfaces. In the pharmaceutical sector, this process is enforced by guidelines like the EU’s Directive 2001/83/EC, which necessitates testing to ensure that information is accessible and actionable for laypersons. For medical devices, the testing is in accordance with standards like IEC 62366 and the FDA’s Human Factors Engineering guidance, emphasizing user interactions to reduce errors. The primary objective is to bridge the divide between complicated medical terminologies and the general public, enhancing both patient safety and adherence to prescribed treatments.
The Key Components of the Testing Process
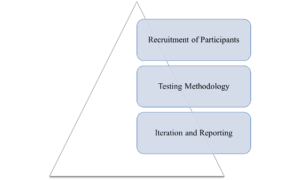
Figure 1: Key Components of Testing Process
- Recruitment of Participants: Typically, 20-30 layman from the target population, including diverse ages and education levels, are chosen, ensuring they are not healthcare professionals to accurately reflect real-world scenarios.
- Testing Methodology: Participants analyze provided materials to find specific information and articulate their understanding through scenarios or quizzes. Interviews and observations reveal pain points, such as confusing layouts or ambiguous language.
- Iteration and Reporting: If comprehension falls below thresholds (80-90% success rate), the material is revised and retested, with a final report submitted to regulators for marketing authorization applications.
This process emphasizes a user-centered approach that identifies critical issues, such as patients misinterpreting dosage instructions, which may result in adverse events.
Why Do Regulators Care About User Testing?
Regulators emphasize the importance of RUT due to its impact on patient outcomes. Inadequate PILs or device instructions can lead to medication errors, non-adherence, and potential harm.

Regulatory User Testing emphasizes “what the patient understands” over “what the company says,” aligning with ICH guidelines and ISO 14971 for risk management.
Why Are Regulators Caring More Than Ever?
In recent years, the scrutiny on user testing has intensified due to several evolving factors:
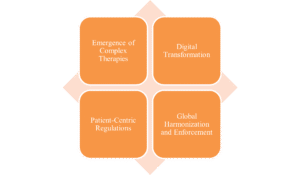
- Emergence of Complex Therapies: With biologics, gene therapies, and personalized medicines, instructions are more intricate. Regulators demand proof that patients can navigate this complexity safely.
- Digital Transformation: The boom in health apps, wearables, and e-labeling post-COVID has expanded RUT to digital interfaces, where usability issues can amplify misinformation or data privacy concerns.
- Patient-Centric Regulations: Initiatives like the FDA’s Patient-Focused Drug Development and EMA’s emphasis on real-world evidence highlight the need for materials that empower patients, not confuse them.
- Global Harmonization and Enforcement: Stricter audits, such as those under the EU’s In Vitro Diagnostic Regulation (IVDR), mandate usability testing for self-testing devices, with non-compliance leading to market barriers.
As a result, failing to prioritize Regulatory User Testing can delay product launches or invite regulatory actions, making it a strategic imperative for pharma and medtech companies.
Zenovel Can Help with Regulatory User Testing-How?
Zenovel specializes in regulatory affairs and GxP services for the life sciences industry, providing comprehensive end-to-end Regulatory User Testing services.

Zenovel specializes in clinical development and strategic consulting, aiming to help clients exceed regulatory expectations and create safer, patient-friendly products.
Regulatory User Testing is essential for patient-centered innovation in pharmaceuticals and medical devices. With increasing regulatory scrutiny, investing in thorough testing is both a compliance necessity and a competitive advantage. Collaborating with experts like Zenovel enhances the approval process and ensures materials are effective in practice. Prioritizing usability is crucial for future health outcomes.