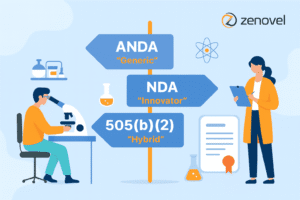
Understanding Drug Approval Pathways: How Regulatory Affairs Services Guide ANDA, NDA & 505(b)(2)
In the pharmaceutical industry, particularly in India’s expanding market, the regulatory pathway is a crucial determinant that influences the transition