
Need of Good Clinical Practice (GCP) Compliance for Clinical Trial Success
In clinical research, the success of drug approval versus regulatory setbacks hinges on the quality and integrity of the data generated Central to this quality assurance is Good Clinical Practice (GCP), which establishes international ethical and scientific standards for the design, conduct, recording, and reporting of clinical trials. GCP serves not only as a regulatory requirement but also as a crucial framework that safeguards human subjects, ensures the credibility of data, and ultimately influences whether effective therapeutic options are made available to patients.
At Zenovel, the company emphasizes its strong reputation built on excellence in GCP, backed by over years of accumulated expertise and experience servicing numerous clients globally. Our GCP services are comprehensive, covering the entire clinical development lifecycle, from Phase I to Phase IV trials, as well as bioequivalence studies and patient-based pharmacokinetic monitoring. The blog discusses the essential aspects of GCP with a focus on independent monitoring, explaining its significance and importance in the context of clinical trials, particularly in 2026.
Considering Good Clinical Practice: Fundamental Framework for Ethical Practice
GCP outlined in the International Council for Harmonisation (ICH) E6 guideline and recently updated to ICH E6 (R3), sets forth standardized protocols for clinical trials across various regions, including the European Union, Japan, Switzerland, and the United States. This framework aims to ensure the mutual acceptance of clinical data by regulatory authorities globally.
The core principles of GCP include:
| Fundamental Aspect
|
What does it means? |
| Ethical Conduct | Trials should adhere to the Declaration of Helsinki, prioritizing the rights, safety, and well-being of subjects above all.
|
| Risk-Based Approach | The monitoring and data collection in a trial should correspond to the associated risks.
|
| Quality by Design | Quality must be ingrained in the scientific and operational design of the trial from the outset, rather than being merely assessed after completion.
|
| Data Accuracy | Records must adhere to the ALCOA+ principles, meaning they should be attributable, legible, contemporaneous, original, accurate, and complete.
|
| Accountability | All participants in the trial are required to possess the necessary education, training, and experience to effectively perform their assigned roles.
|
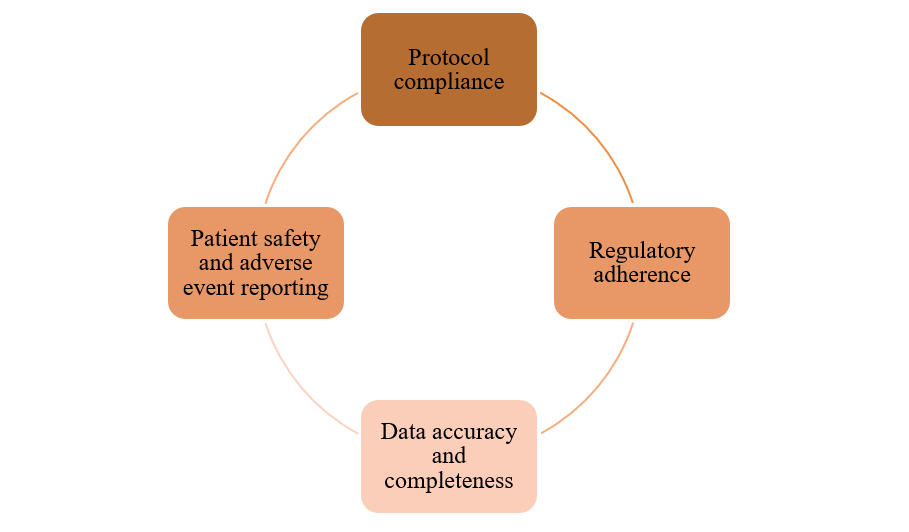
Compliance with GCP is mandatory, and regulatory bodies like the US FDA, EMA, MHRA, and India’s CDSCO perform Bioresearch Monitoring (BIMO) inspections to ensure adherence. Non-compliance may lead to warnings, clinical holds, data rejection, and disqualification of investigators.
What Is Independent Monitoring?
Independent monitoring involves oversight of a clinical trial by unbiased individuals or groups without interests in the trial’s results, ensuring objective assessments separate from the sponsor’s teams and investigative sites.
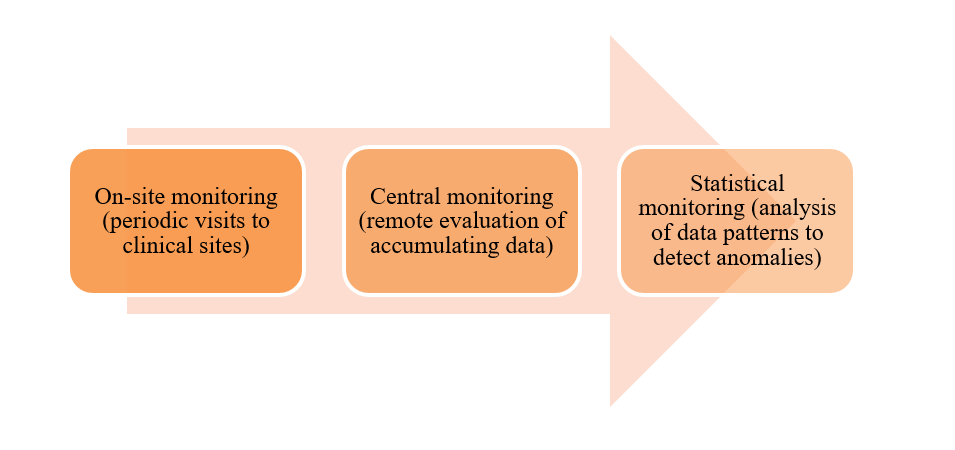
The ICH E6 (R3) guideline mandates that sponsors establish a monitoring system tailored to the trial’s risks, potentially utilizing a mix of strategies.
Types of Independent Monitoring Bodies
Different monitoring structures may be appropriate depending on the trial’s phase, size, and risk profile.
- Independent Medical Monitor: is a qualified physician who evaluates adverse events in clinical trials and advises on the safety of continuing the study, particularly in small, low-risk trials.
- Safety Monitoring Committee: is an independent group of experts responsible for regularly reviewing adverse event data, though they generally do not conduct interim efficacy evaluations. They may be appointed at the beginning of a trial or called together as necessary.
- Data and Safety Monitoring Board (DSMB) or Independent Data Monitoring Committee (IDMC): acts as the primary oversight body for Phase III, multi-site clinical trials. Their responsibilities encompass interim efficacy analyses, reviews of ancillary studies, and recommendations for protocol modifications. DSMBs typically comprise clinicians with specialized knowledge in the relevant disease and at least one independent statistician.
Why Independent Monitoring Matters?
The importance of independent monitoring cannot be overstated. Here’s why it is indispensable to modern clinical research:
Patient Safety Protection
The primary obligation of clinical trials is to protect participants. Independent monitors provide an unbiased review of safety data, identifying trends that internal teams may overlook. According to the NHMRC Clinical Trial Centre, Independent Data Monitoring Committees (IDMCs) assess trial data to ensure compliance with scientific, regulatory, and ethical standards, and make recommendations for patient safety.
Data Integrity and Fraud Detection
Data integrity challenges stem from unintentional errors and intentional fraud, with statistical monitoring being effective in detecting both issues.
Fraud detection through statistical monitoring focuses on:
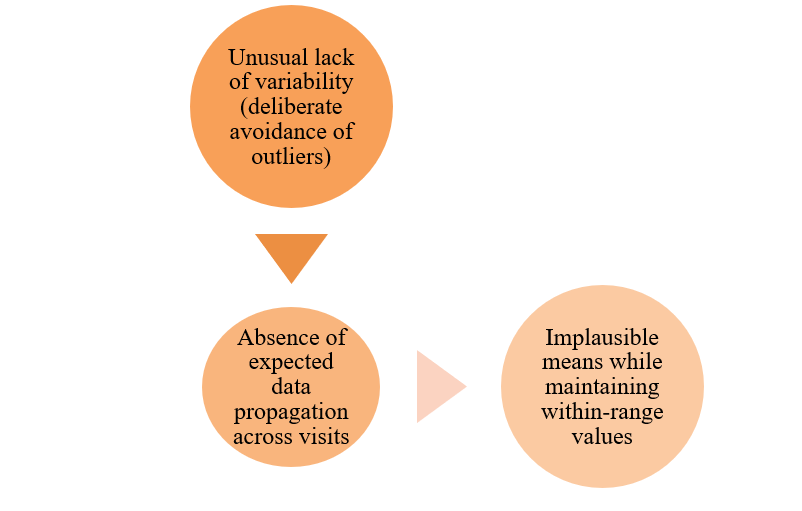
Statistical monitoring is now essential as the ICH E6 (R3) guideline mandates the identification of “potential data manipulation and data integrity problems.”
Regulatory Compliance and Inspection Readiness
Regulatory authorities keep comprehensive records of investigator compliance, with the FDA’s Bioresearch Monitoring Information System (BMIS) cataloging details about clinical investigators, CROs, and Institutional Review Boards in IND studies. The Clinical Investigator Inspection List monitors inspection results, where serious violations may result in Notice of Initiation of Disqualification Proceedings (NIDPOE) letters. Independent monitoring serves as evidence that sponsors have met their regulatory responsibilities in overseeing trial conduct, which is crucial during regulatory inspections.
Unbiased Interim Analysis
Independence of the monitoring body is essential in trials with interim analyses to prevent bias from sponsors with access to unblinded results. The Statistical Data Analysis Center (SDAC) serves as an independent entity that prepares unblinded reports for the Independent Data Monitoring Committee (IDMC) while ensuring sponsors remain blinded to treatment group results, thereby preserving the scientific integrity of ongoing trials.
Early Termination Decisions
Some trials may need to be halted due to harmful interventions, ethical concerns regarding continued placebo treatment in light of clear efficacy, or indications of futility in interim data. Independent monitoring bodies can suggest early termination in these cases, helping to safeguard patient welfare and potentially save sponsors considerable costs.
- Source Data Verification (SDV)
Traditional monitoring approaches that utilized 100% Source Data Verification (SDV) involved exhaustive reviews of all data points against source documents, demanding frequent on-site visits. Although thorough, this method is resource-intensive, costly, not proportionate to risk by giving equal weight to critical and non-critical data, and slow to detect systemic issues.
- Risk-Based Monitoring (RBM):
Risk-Based Monitoring (RBM) signifies a significant change promoted by the FDA and ICH E6 (R2/R3), emphasizing the allocation of monitoring resources to the most significant risks impacting data integrity and patient safety.
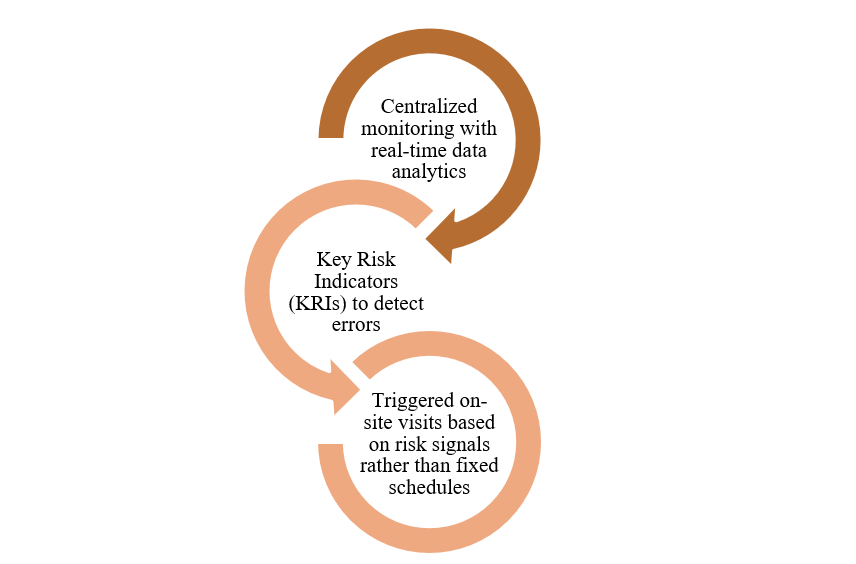
RBM signifies a significant change promoted by the FDA and ICH E6 (R2/R3), emphasizing the allocation of monitoring resources to the most significant risks impacting data integrity and patient safety.
Zenovel offers tailored independent monitoring services for trials, supported by GCP experts with decades of experience, and is based in Ahmedabad with a global presence.
Our GCP Service Portfolio
Expertise of Zenovel for Independent Monitoring
- Our regulatory affairs team manages submissions and compliance with key authorities such as USFDA, EMA, MHRA, WHO, ANVISA, MCC, and TGA.
- Our documentation control adheres to ALCOA+ principles, ensuring that all records are attributable, legible, contemporaneous, original, and accurate.
- Global Delivery, Local Presence: Located in India’s top pharmaceutical hub, we provide affordable excellence while maintaining rigorous quality and regulatory standards.
- Our team possesses expertise in various therapeutic areas, including ophthalmology, oncology, immunology, cardiology, medical devices, and diverse study designs.
Ready to Strengthen Your Trial Oversight? Contact Zenovel’s GCP Services Team today to discuss how our independent monitoring solutions can protect your trial’s integrity and accelerate your path to approval.
References:
- NIGMS Guidance on Data and Safety Monitoring
- FDA Clinical Investigations Compliance & Enforcement
- Applied Clinical Trials: Detecting Fraud Using Statistical Monitoring