
In the pharmaceutical and biotechnology industries, obtaining regulatory approval is essential prior to introducing new drugs or biologics to the market. A pivotal aspect of this approval process is the FDA Pre-Approval Inspection (PAI), which involves a comprehensive assessment of manufacturing facilities, processes, and data integrity to ensure adherence to Current Good Manufacturing Practices (cGMP). Successfully passing the Pre-Approval Inspections (PAI) is crucial for the success of a submission, yet many companies encounter difficulties in effective preparation for this evaluation.
This highlights the importance of regulatory affairs services and compliance consultants in facilitating market entry for companies. Regulatory pre-submission consulting and Pre-Approval Inspections (PAI) readiness services are essential in identifying and addressing potential gaps, which contributes to smoother inspections. Zenovel exemplifies companies that specialize in regulatory submission services and FDA pre-approval inspection preparation, providing indispensable expertise to navigate the complexities of global regulations.
Understanding Pre-Approval Inspections (PAI)
The FDA conducts PAIs to verify that:
FDA Pre-Approval Inspections
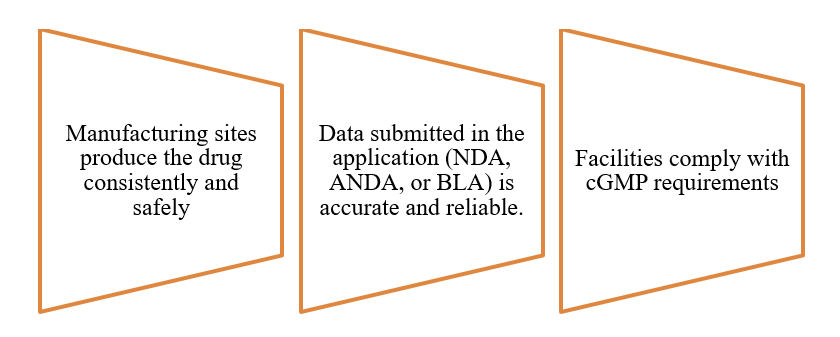
Inspections examine quality systems, process validation, laboratory controls, and documentation. Identified deficiencies may result in Form 483 observations, delays, or Complete Response Letters. Preparation begins prior to the inspection notification, typically during the pre-submission phase.
The Role of Regulatory Affairs Pre-Submission Services
Regulatory pre-submission consulting aids in early strategic planning to meet regulatory expectations, ensuring alignment between development and inspection readiness while avoiding unexpected issues.
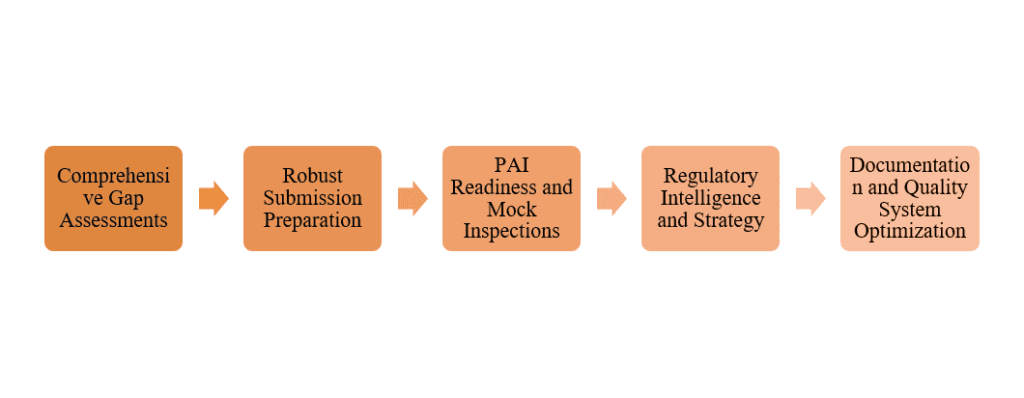
Key ways pre-submission services support successful PAIs:
Ways for pre-submission services
Comprehensive Gap Assessments:
Experts perform mock audits and gap analyses to pinpoint cGMP compliance issues in facilities, processes, and documentation, enabling corrective actions prior to FDA inspections.
Robust Submission Preparation:
Regulatory submission services guarantee that dossiers, such as CMC sections, are thorough, precise, and prepared for inspection, which involves validating manufacturing processes and maintaining data integrity.
PAI Readiness and Mock Inspections:
Pre-Approval Inspections (PAI) readiness services encompass simulated inspections, training for site staff, and the development of response strategies, all aimed at building confidence and refining procedures through drills.
Regulatory Intelligence and Strategy
Staying ahead of evolving guidelines (FDA, EMA, etc.) helps anticipate inspector focus areas, such as quality risk management or supply chain controls.
Documentation and Quality System Optimization
Consultants strengthen quality management systems (QMS), SOPs, and record-keeping to withstand scrutiny.
Partner with Zenovel-A Regulatory Compliance Consultant
Zenovel is a leading provider of regulatory affairs services, specializing in supporting pharmaceutical and biotech companies with our integral , focusing on GCP, GMP, and regulatory compliance.
Zenovel Services
Zenovel’s team possesses substantial regulatory expertise and practical experience, establishing them as a valuable partner for FDA pre-approval inspection preparation and PAI readiness services. Our approach to regulatory pre-submission consulting effectively transforms potential pitfalls into strengths, allowing companies to engage experts early in the process. This proactive strategy minimizes inspection risks, accelerates approval times, and facilitates the faster market entry of innovative therapies, all while maintaining a strong focus on patient safety and compliance.
Whether you’re preparing for an NDA, ANDA, or global submission, proactive regulatory support is essential. Ready to strengthen your pre-approval inspection (PAI) support strategy? Contact Zenovel today to explore our regulatory affairs services and ensure your next inspection is a success.