In clinical research, Good Clinical Practice (GCP) sets ethical and quality standards. Non-compliance during audits poses systemic risks to patient safety and data integrity, threatening trial validity. As leading GCP consultants in Ahmedabad often observe, traditional Corrective and Preventive Action (CAPA) methods fail because they address only surface-level symptoms rather than systemic root causes—highlighting the need for a modern, strategic approach.
Why Traditional CAPA Often Fails in GCP?
Many organizations view Corrective and Preventive Action (CAPA) as merely a documentation task, often reduced to completing forms for audit compliance. However, this approach overlooks the more critical aspects of CAPA’s function and can lead to significant pitfalls.
- Root Cause Stopping at “Human Error”: Labeling protocol deviations or inadequate informed consent as “staff error” is unproductive; instead, we should investigate the underlying systems, processes, or training gaps that permitted the error to happen.
- Isolated Corrections: Fixing only the immediate issue without considering other trials, sites, or processes where the same weakness might exist is inadequate.
- Preventive Actions as an Afterthought: Preventive actions that are weak and generic, such as “retrain staff,” fail to address the fundamental flaws in the underlying processes.
- Lack of Effectiveness Checks: Failure to verify, using data and metrics, whether the CAPA was effective and did not introduce new risks.
The Modern CAPA Framework: Strategic, Systemic, and Sustainable
A modern CAPA process is a powerful quality management tool. Here’s a proactive approach:
- Robust Root Cause Analysis (RCA): Going Beyond the 5 Whys
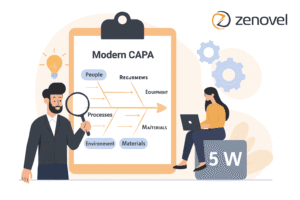
- Utilize Fishbone Diagrams: Visually categorize causes into six groups: People, Processes, Equipment, Materials, Environment, and Management.
- Apply Pareto Analysis: Identify the key systemic issues that account for the majority of non-compliances in order to prioritize strategic interventions effectively.
- Action Design: Corrective vs. Preventive
- Corrective Actions: Actions should be prompt and specifically tailored to rectify the situation, such as correcting data entries or filing missed reports.
- Preventive Actions To enhance the monitoring process, it is crucial to revise the monitoring plan template by adding a mandatory verification step for informed consent documentation and to implement a centralized tracker for site communication.
- Effectiveness Verification: The Proof is in the Data
A CAPA is considered closed not when the action is implemented, but only after its effectiveness is demonstrated.
- Clear metrics should be defined, such as reducing protocol deviations in this category by 50% during the next two monitoring visits.
- Future review and analysis will be scheduled.
- Using audit findings, quality metrics, and site performance data as evidence.
- Knowledge Management: Leveraging Lessons Learned
A modern Corrective and Preventive Action (CAPA) system enhances organizational learning by analyzing insights from one CAPA for potential application across various studies and departments, thereby preventing recurrence on a global scale.
The Strategic Advantage of Expert-Led CAPA Management
Implementing this modern approach necessitates deep GCP expertise and a quality mindset, where collaboration with specialists can turn a regulatory obligation into a competitive advantage.
The right experts help you:
- Develop CAPAs that satisfy regulatory standards and showcase a robust quality culture.
- Integrating CAPA with Risk-Based Quality Management (RBQM) transforms findings into proactive risk controls.
- Ensuring trial data is audit-ready is essential for protecting investments by safeguarding regulatory submissions and facilitating product approvals.
Zenovel is recognized as a leading GCP consultants in Ahmedabad, known for its strategic and pragmatic approach to addressing GCP non-compliance in clinical research organizations, sites, and sponsors both locally and across India.
Our Modern CAPA Services Include:
- GCP Compliance Audits & Mock Inspections: Non-compliances are identified with the necessary clarity and depth for effective root cause analysis (RCA).
- Facilitated Root Cause Analysis Workshops: We help teams identify and address underlying systemic causes instead of attributing issues to “human error.”
- Strategic CAPA Plan Development: We assist in creating robust, effective, and verifiable corrective and preventive actions.
- CAPA Effectiveness Review & Metrics Design: We establish the framework to demonstrate the effectiveness of your solutions.
- GCP Training & Quality Culture Building: We empower your team to prevent non-compliance at its source.
Modernize your Corrective and Preventive Action (CAPA) strategy to improve quality, enhance patient safety, and protect data. Shift your GCP quality management from reactive to strategic by partnering with Zenovel.
Contact top GCP consultants in Ahmedabad to create a compliant clinical research operation.