Risk Based Monitoring (RBM) is a more efficient alternative to traditional clinical trials, focusing on high-risk areas through data-driven insights. It ensures patient safety, data integrity, and regulatory compliance while reducing costs and timelines. However, the success of RBM depends on the competency of monitors, who identify risks, analyze data, and implement mitigation strategies.
At Zenovel, we assists pharmaceutical and biotech clients in implementing Risk Based Monitoring , offering customized training, tools, and quality assurance frameworks. Drawing on industry best practices and regulatory guidance from bodies like FDA and EMA, they provide training and competency for RBM monitors, assisting clients transitioning from traditional monitoring or optimizing existing setups.
Compulsory and Essential Skills for RBM Monitors
RBM monitors require a combination of technical, analytical, and soft skills to excel in a data-centric environment, prioritizing centralized oversight, risk detection, and targeted interventions, setting top RBM monitors apart.
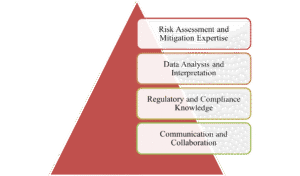
- Risk Assessment and Mitigation Expertise: Monitors must identify critical data points and processes that could impact trial outcomes, such as patient safety metrics or endpoint data from inexperienced sites. Risks are categorized using tools like RACT and visualized using ‘traffic light’ systems. Training focuses on drawing insights from past studies to flag potential issues early and adjust mitigation strategies dynamically.
- Data Analysis and Interpretation: RBM focuses on detecting anomalies in trial data. Monitors should be proficient in interpreting lab data and identifying necessary patterns. This skill set helps identify issues like training gaps, errors, or potential fraud.
- Regulatory and Compliance Knowledge: The FDA and EMA have advised RBM for all trial phases, requiring monitors to stay updated on monitoring plans, escalation protocols, and quality management, ensuring lower-risk studies require less data review and aligning activities with good clinical practice.
- Communication and Collaboration: RBM success necessitates efficient collaboration with stakeholders like sponsors, CROs, site staff, statisticians, and programmers, with monitors ensuring clear communication, timely issue resolution, and motivational support.
Zenovel offers trainings and regulatory assistance needed for the client’s staffs, enhancing their monitors’ knowledge and confidence in applying these skills to real-world scenarios.
Key Tools in Risk Based Monitoring
RBM relies on technology for real-time data analysis and centralized monitoring, with monitors trained on tools for signal detection, risk visualization, and efficient oversight, based on established frameworks.
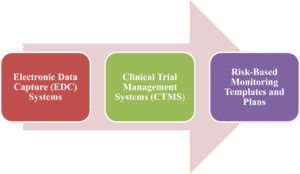
- Electronic Data Capture (EDC) Systems: Platforms organize trial data from various sites, enabling targeted SDV on high-risk elements, with training in query resolution and integration with other systems for comprehensive oversight.
- Clinical Trial Management Systems (CTMS): CTMS tools offer dashboards for monitoring enrollment, compliance, and site performance, identifying outlier data, facilitating centralized monitoring, and reducing onsite visits.
- Risk-Based Monitoring Templates and Plans: The document provides SDV/SDR thresholds, risk levels, trial phases, and risk categorization tables for potential risks, such as safety and data integrity.
Zenovel assists clients in selecting and implementing tools, providing hands-on training for integration into monitoring workflows, ensuring proactive risk management and improved trial efficiency.
Quality Assurance in Risk Based Monitoring
Quality assurance (QA) is crucial in Resource-Based Management (RBM) to ensure data integrity and optimize resources through structured processes for monitoring, evaluating, and refining activities.
- Comprehensive Monitoring Plans: FDA guidelines mandate a trial monitoring plan (TMP) with methods, responsibilities, data points, frequency, and communication strategies, with quality assurance training emphasizing risk assessments and incorporating centralized, remote, or targeted strategies.
- Metrics, Reports, and Escalation Procedures: Customized metrics track KRIs and CDEs, while QA procedures involve data cleaning, quality control, and timely escalation to resolve issues without disrupting the trial.
- Ongoing Evaluation and Training: RBM requires continuous monitoring and adjustments based on feedback, including competency assessments and retraining on evolving tools and regulations, to ensure its effectiveness.
Zenovel addresses common challenges like Risk Based Monitoring adoption due to unfamiliarity with QA processes by providing QA consultancy, change management support, and trust building to ensure compliance without compromising efficiency.
How Zenovel Helps Clients Implement RBM Training and Competency
Zenovel provides comprehensive support for clients transitioning to Risk Based Monitoring, addressing concerns about data quality and team readiness.
- Design Customized Training Programs: Our programs provide monitors with the necessary skills for success, incorporating real-world case studies, regulatory guidance, and industry trends like the rising adoption of RBM.
- Provide Tool Integration and Consultancy: Zenovel experts assist in selecting, setting up EDC, CTMS, and analytics tools, ensuring seamless integration with existing systems, and developing customized monitoring plans and risk templates for trial specifics.
- Ensure Robust Quality Assurance: Our Quality Assurance frameworks utilize risk assessments, escalation protocols, and ongoing evaluations to minimize challenges, maximize benefits like cost savings and improved data quality.
Overall, Zenovel’s partnership helps clients meet regulatory expectations and expedite approval times for investigational products, benefiting sponsors, CROs, and site teams by empowering monitors and streamlining operations. Effective Risk Based Management (RBM) involves training and competency to transform monitors into strategic risk managers. With the right skills, tools, and QA practices, Risk Based Monitoring can revolutionize clinical trials by reducing costs, enhancing data quality, and prioritizing patient safety. Zenovel is committed to helping clients navigate this shift confidently.
Book your request for robust and skillful RBM monitors for your next project on bd@zenovel.com.